Research
Ambient-Temperature Serial Femtosecond X-ray Crystallographic Studies of Ribosome Complexes
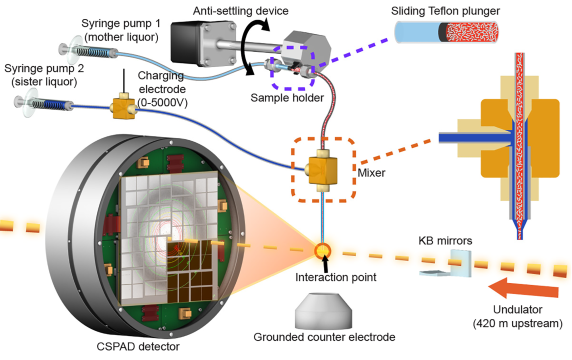
High-resolution ribosome structures determined by cryo X-ray crystallography have provided important insights into the mechanism of translation. Such studies have thus far relied on large ribosome crystals kept at cryogenic temperatures to reduce radiation damage. We use the serial femtosecond X-ray crystallography (SFX) with an X-ray free-electron laser (XFEL) to obtain diffraction data from ribosome microcrystals in liquid suspension at ambient temperature. Small 30S ribosomal subunit microcrystals programmed with decoding complexes and bound to either antibiotic compounds or their next-generation derivatives diffracted to high resolution. Our results demonstrate the feasibility of using SFX to better understand the structural mechanisms underpinning the interactions between ribosomes and other substrates such as antibiotics and decoding complexes.
We have determined the structure of large (50S) ribosomal subunit in record-short time by using record-low amount of sample during and XFEL beamtime . This structure is the largest one solved to date by any FEL source to near atomic resolution (3 MDa). We expect that these results will enable routine structural studies, at near-physiological temperatures, of the large ribosomal subunit bound to clinically-relevant classes of antibiotics targeting it, e.g. macrolides and ketolides, also with the goal of aiding development of the next generation of these classes of antibiotics. Overall, the ability to collect diffraction data at near-physiological temperatures promises to provide new fundamental insights into the structural dynamics of the ribosome and its functional complexes.